MEDICAL COMPOUNDS PRODUCED IN A CLEAN ROOM
With our MARFRAN.MED we supply the pharmaceutical and medical industry, producing in a controlled environment, in accordance with ISO 9001 and ISO 13485 and the ISO 14644 standard.
The materials are basedon both styrenic block copolymers (TPE-SEBS) and polyolefins (TPO).
The high quality of MARFRAN.MED allows to create products:
1. Highly flexible
2. With rubber-like appearance and feeling
3. PVC-free, phthalate-free, silicon-free and latex-free in the whole production chain
4. Highly transparent and easy to color
5. Recyclable
6. Sterilizable with gamma rays, EtO and steam (variable according to grades)
We produce according to ISO 90001 and ISO 13485 standards (which regulate the production process of medical products) in a new clean room specifically built, highly automatized and subjected to cleaning and management procedures.
We have choosen the best technology to provide our clients the best quality level: our compounds must undergo a strict selection process of the finale granule through an optic system.
We select medical raw materials to create the MARFRAN.MED TPE compound, and we guarantee the reliability of both the formulation and the sources of raw materials.
We also guarantee a two-year “Notification of Change" in case a change in supplier, raw material or formulation is necessary due to extenuating circumstances.

Each family of compound underwent biocompatibility tests according to the following regulations.
ISO 10993 (for biocompatibility, safety and implantability of medical devices), and more specifically:
• ISO 10993-4 Hemolysis
• ISO 10993-5 Cytotoxicity
• ISO 10993-10 Irritation and skin sensitization
• ISO 10993-11 Systemic toxicity
USP Class VI (quality, pureness, strength and consistency standards of American pharmacopeia), and more specifically:
• Intracutaneous test
• Systemic injection test (Acute Systemic Toxicity)
• Implantation test
We satisfy the highest requirements of Class VI because of the low level of toxicity of our compounds, thanks to our production environment with controlled contamination and the accurate selection of medical raw materials.
The range of MARFRAN.MED products finds application in the production of items dedicated to the medical and pharmaceutical industry:
infusion and transfusion lines, drainage bags, catheters, components for hemodialysis and urodynamic devices, devices for oxygen therapy, surgical instruments, tubes for peristaltic pumps, micro-balloons for catheters, breathing aids, individual protection masks, fittings, membranes elastics, dispensers, devices for pressure gauges, gaskets, products for orthopedics, containers for analyzes, valves… and many other items.
Don’t hesitate to contact us to find out more about it!
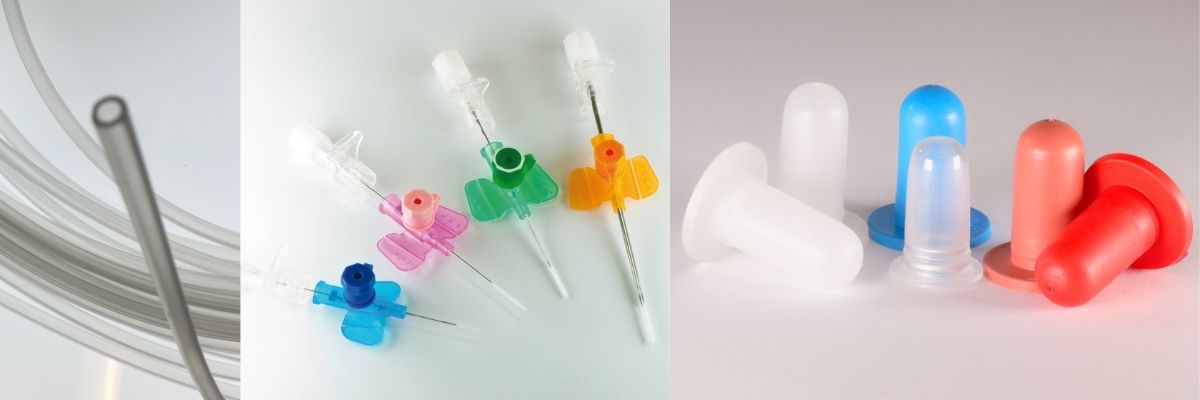
The MARFRAN.MED compounds can be processed through extrusion, co-extrusion, injection or bi-injection molding, blow-molding, micro-extrusion, and micro-molding processes.
Our products range includes materials that can adhere to Polyolefine, and special grades that stick to polymers such as PC, ABS, SAN, and ASA.
R&D Department can work together with designers and technicians in order to develop customized compounds dedicated to specific applications.
For more information about MARFRAN.MED please fill in the form below or write to: info@marfran.com
